Organic Molecules
Fluorescence and fluorescence optical microscopy are widely used to evaluate organic molecules, including extensive use in imaging photoluminescent dyes in labeled biological cells and tissues. However, the spatial resolution is restricted to ~500 nm because of the optical diffraction limit when using visible light. In principle, the electron beam can overcome this limitation by exciting cathodoluminescence (CL) with nano-sized probes.
Luminescence is generated based on the relaxation of electrons from the lowest unoccupied molecular orbital (LUMO) to the highest occupied molecular orbital (HOMO), analogous to the band gap of inorganic crystals. The transition of electrons across the HOMO-LUMO energy gap can result in the emission of characteristic photons in the visible wavelength range, enabling wavelength spectroscopy to differentiate organic molecules and map their distribution with nanoscale resolutions. Unlike fluorescence microscopy, the incident electron beam can be considered a broadband excitation source without the limitation of needing to match the incident wavelength with the absorption spectra of the studied material.
CL imaging of biological samples is extremely challenging due to technological limitations in the efficient collection of usually very low-intensity optical signals and quenching of signals as many organic molecules disintegrate under the electron beam. Nevertheless, CL is an attractive tool for several applications:
Biological Specimens
Electron microscopy is instrumental in the development of our understanding of biological systems. Although electron microscopy reveals ultrastructure with nanoscale resolution, it does not provide the same information as optical fluorescence microscopy regarding the location of proteins within a cellular context. Many researchers seek to extend cathodoluminescence (CL) to the biological sciences to form a direct correlation of protein location and function with the ultrastructure. Success using traditional (optical) fluorophores has been limited due to the damage caused by the electron beam. Notwithstanding, researchers use CL successfully to image Alexa 488 to determine the roles of the targeted monocytes in cell growth.
Other researchers want to develop and deploy multi-colored inorganic, electron-hard CL tags for optical and electron microscopes, such as nano-diamonds or -phosphors. For example, early results based on lanthanide-doped ceramic nanoparticles suggest that optimizing nanoparticle composition, synthesis protocols, and electron imaging conditions can enable high signal-to-noise localization of biomolecules with a sub-20-nm resolution, limited only by the nanoparticle size.
Pharmaceuticals
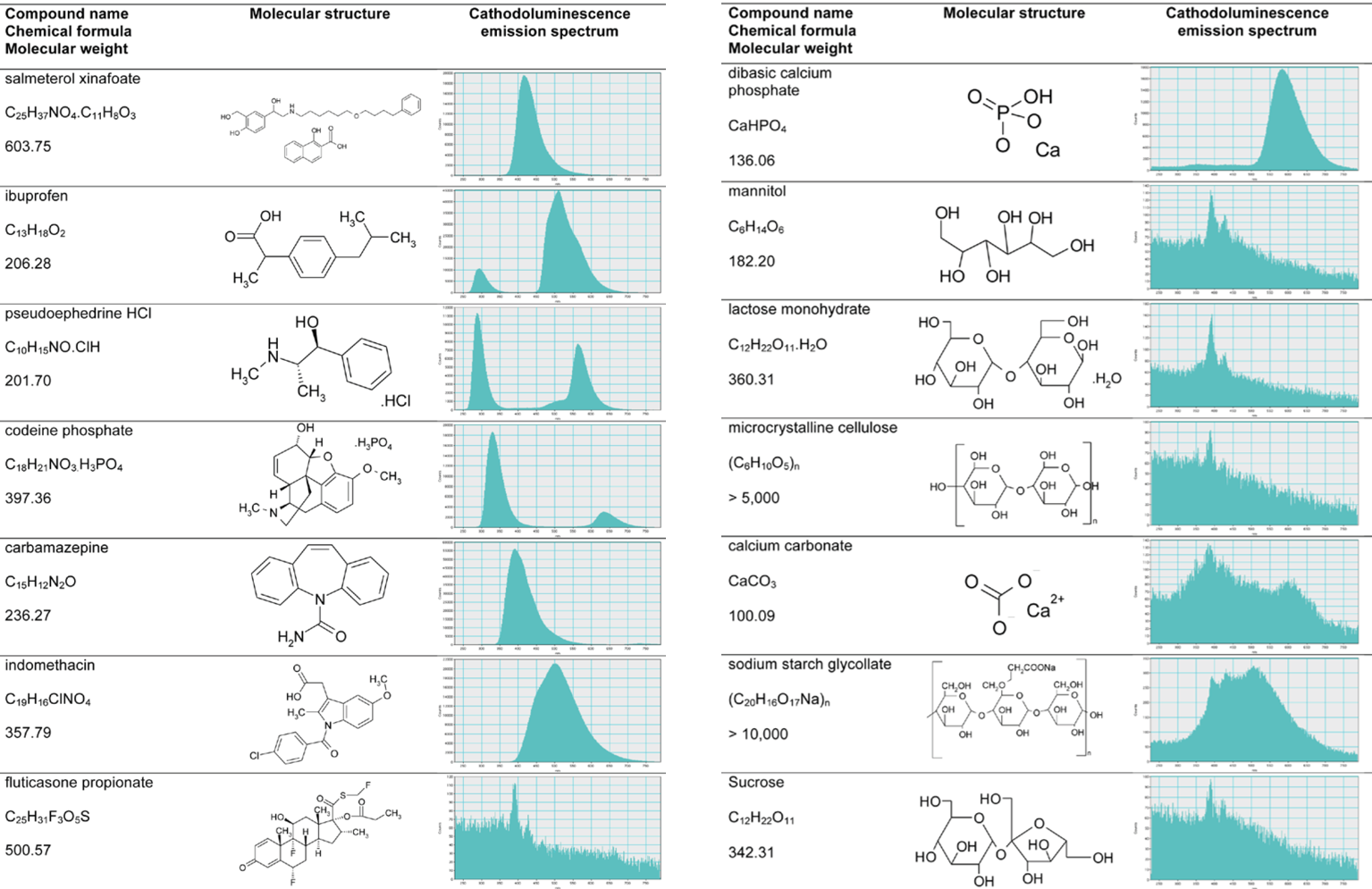
Cathodoluminescence (CL) analysis complements other spectroscopic and imaging tools researchers use to understand, develop, and manufacture solid-dosage medicinal products. The luminescence signatures of an organic molecule stem from the molecule's chemical structure (HOMO-LUMO gap) rather than sample composition. Up to 80% of active pharmaceutical ingredients (APIs) strongly exhibit CL in the scanning electron microscope compared to only 20% of excipients.
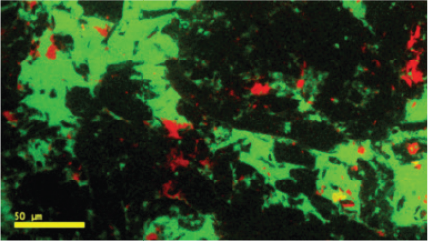
Due to the different crystal structures, researchers also use CL to distinguish the solid forms of some drug compounds. For example, the four polymorphs of carbamazepine and the crystalline and amorphous forms of indomethacin. These observations enable the identification of changes to the solid form of an API after it is formulated into a tablet, pellet, or blend.
Experimental briefs and application notes
 |
A new role for cathodoluminescence spectroscopy and imaging as an analytical tool to support the development of pharmaceutical products |
Polymers
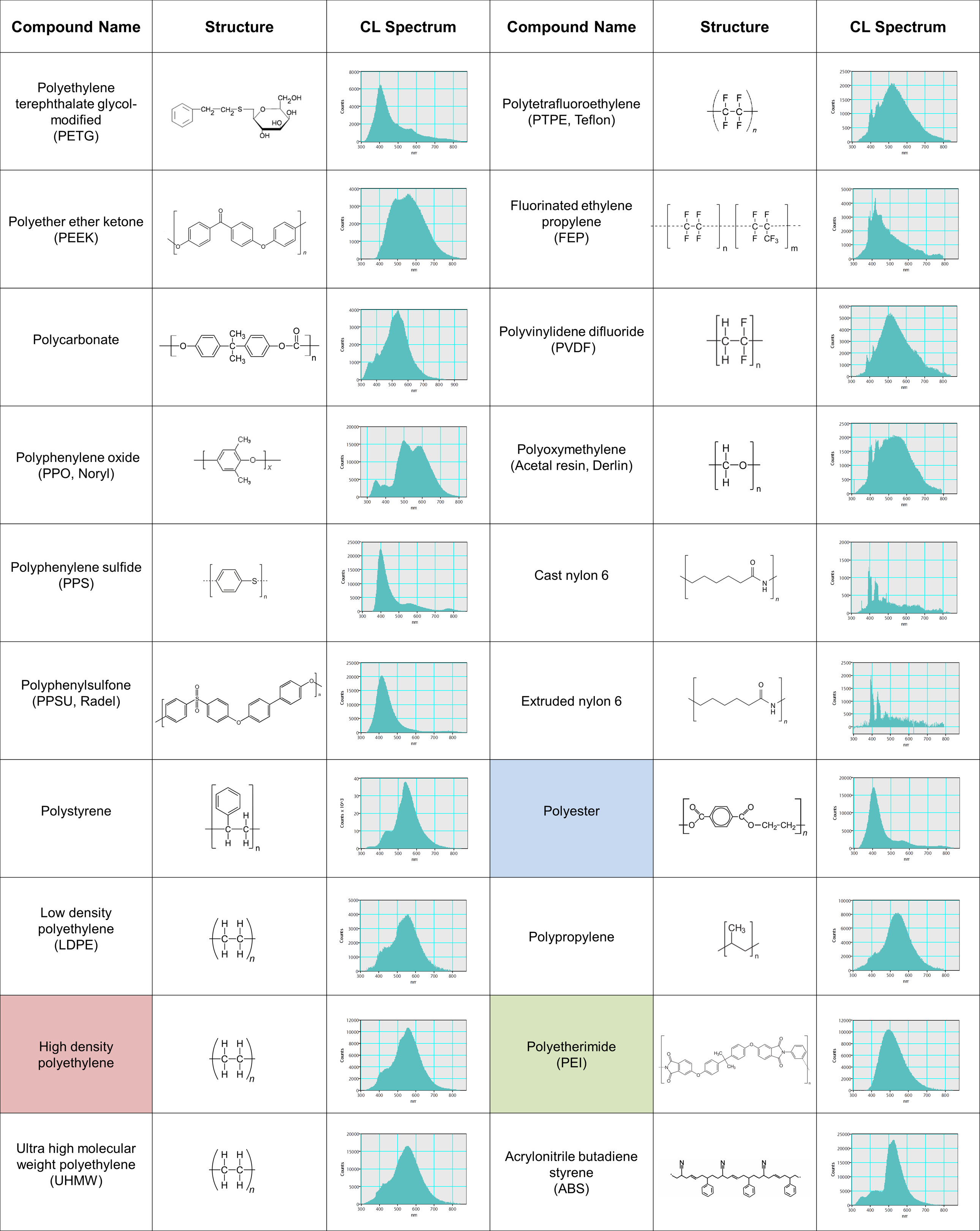 Revealing the distribution of materials at the spatial resolution provided by a scanning electron microscope is important in many applications, including composite materials—where physical properties critically depend on component distribution and morphology—and foreign body analysis. In the case of organic materials, standard imaging and analysis techniques are often unable to identify and distinguish materials unambiguously. An alternative technique that can differentiate materials independent of their elemental composition is necessary in such cases.
Revealing the distribution of materials at the spatial resolution provided by a scanning electron microscope is important in many applications, including composite materials—where physical properties critically depend on component distribution and morphology—and foreign body analysis. In the case of organic materials, standard imaging and analysis techniques are often unable to identify and distinguish materials unambiguously. An alternative technique that can differentiate materials independent of their elemental composition is necessary in such cases.
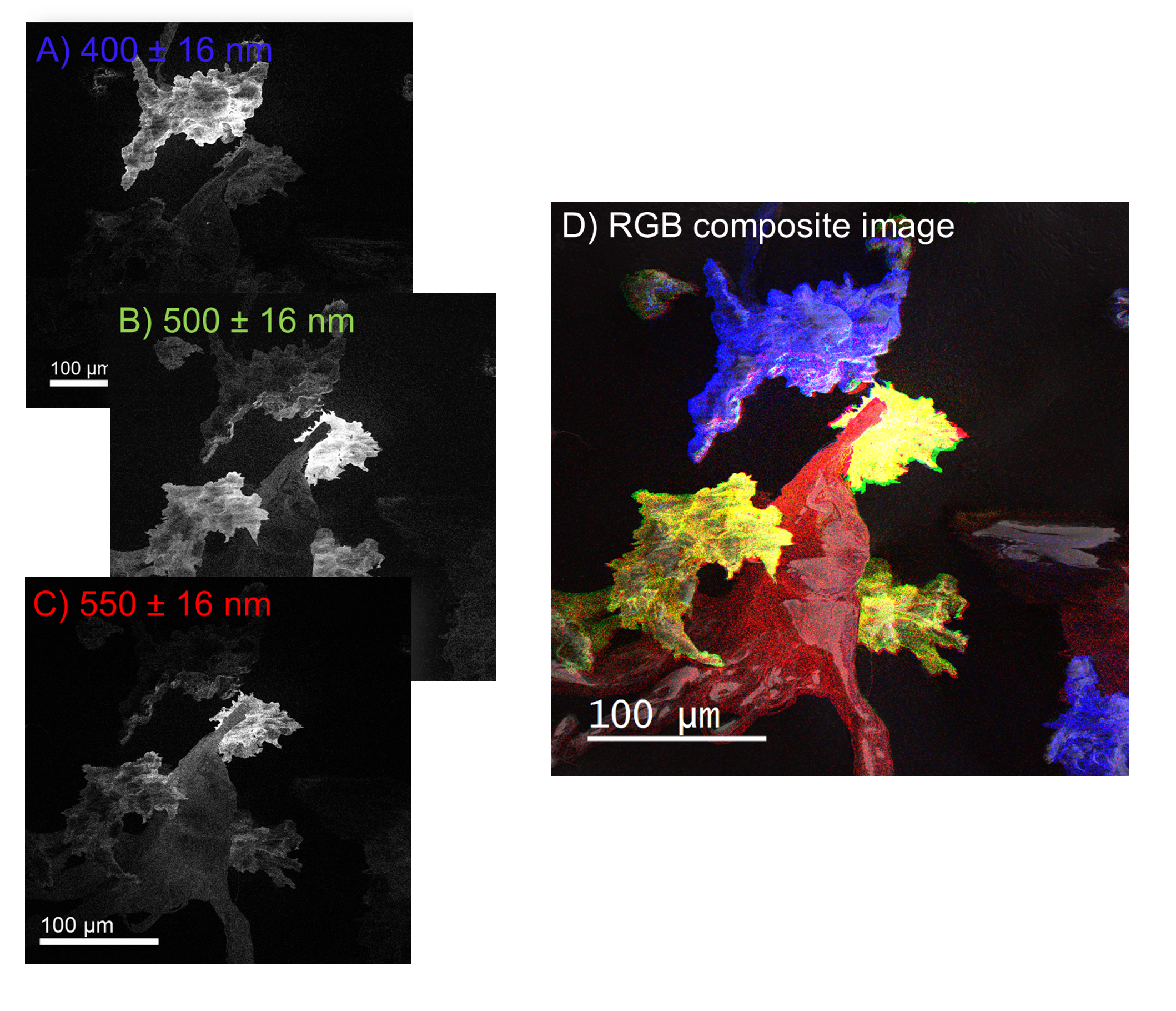
Cathodoluminescence (CL) analysis of organic molecules depends on the molecular structure and inter-molecule interactions rather than elemental composition. Many polymers are cathodoluminescent and are distinguishable based on their wavelength spectrum (see table). As an example, wavelength-filtered CL maps are shown to reveal the distributions of three-component ingredients from a compound specimen: polyester ((C10H8O4)n), high-density polyethylene ((C2H4)nH2), and polyetherimide ((C37H24O8N2)n).