 Oxides and nitrides, such as sapphire (Al2O3) and gallium oxide (Ga2O3), are considered wide band gap insulators. In these materials, the high-energy electron beam of an electron microscope offers an ideal tool to study the electronic and optical properties without resorting to specialized deep ultraviolet lasers for photoluminescence. Cathodoluminescence (CL) helps determine the energy band gap and reveal the presence (and energy state) of electrically active defects. This makes it a preferred tool to study the basic optical properties of these materials and discriminate between different phases of compositionally and chemically identical materials, e.g., the anatase and rutile phases of TiO2.
Oxides and nitrides, such as sapphire (Al2O3) and gallium oxide (Ga2O3), are considered wide band gap insulators. In these materials, the high-energy electron beam of an electron microscope offers an ideal tool to study the electronic and optical properties without resorting to specialized deep ultraviolet lasers for photoluminescence. Cathodoluminescence (CL) helps determine the energy band gap and reveal the presence (and energy state) of electrically active defects. This makes it a preferred tool to study the basic optical properties of these materials and discriminate between different phases of compositionally and chemically identical materials, e.g., the anatase and rutile phases of TiO2.
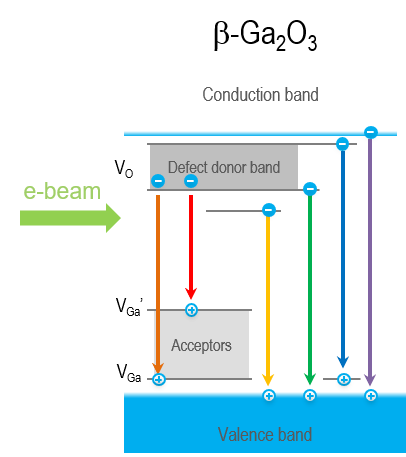
Recently, β-Ga2O3 has received increased attention due to its intrinsic ultra-wide band gap and optical transparency in visible light, implying a natural application to solar-blind ultraviolet (UV) photodetection. Limited techniques exist to determine material quality and characterize the defects that may be present. CL is one such technique—CL spectroscopy has been used to reveal oxygen vacancy-related and (two) gallium vacancy-related energy levels within the β-Ga2O3 band gap, including spectral changes associated with removing or creating these defects through materials processing. These defect levels compete with the near band edge luminescence, and the relative ratio of the two luminescence types provides insight into material quality.
 |
Spectroscopic analysis of ultra-wide bandgap semiconductors |