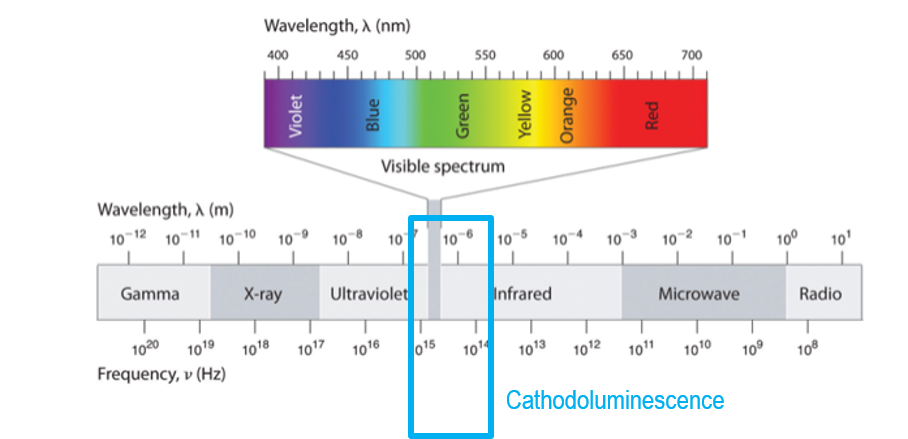
 While luminescence is the emission of light from a solid when excited by an external energy source, cathodoluminescence (CL) is when the energy source is high-energy electrons. Although you may not be familiar with CL, you have undoubtedly seen it. Many people see CL when viewing a display (monitor or television) that uses a cathode ray tube (electron gun) to produce light from a phosphor screen or the green viewing screen of a transmission electron microscope.
While luminescence is the emission of light from a solid when excited by an external energy source, cathodoluminescence (CL) is when the energy source is high-energy electrons. Although you may not be familiar with CL, you have undoubtedly seen it. Many people see CL when viewing a display (monitor or television) that uses a cathode ray tube (electron gun) to produce light from a phosphor screen or the green viewing screen of a transmission electron microscope.
Many materials exhibit CL, including phosphors, semiconductors, ceramics, geological minerals, gemstones, organic compounds, and (some) metallic structures for nanophotonic applications. Analysis of the emitted light can reveal important structural and functional properties of a sample that you cannot often achieve by other methods.
A meeting of optical emission spectroscopies and electron microscopy
Cathodoluminescence microscopy is the term used to describe the analysis of light emitted from a sample in an electron microscope; the light may be in the ultraviolet, visible, and infrared wavelength portions of the electromagnetic spectrum.
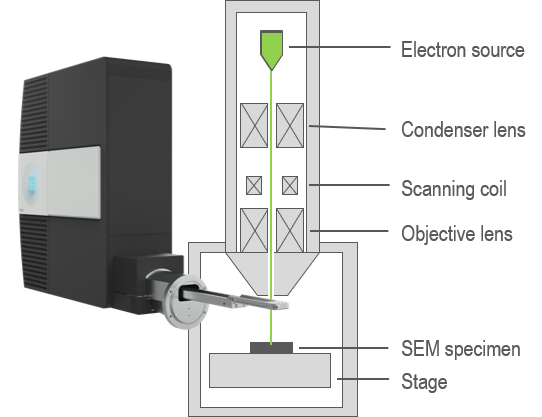 In an electron microscope, you can attain spatially resolved information (images or maps) by scanning a sub-nm diameter electron beam across the surface of a sample. By collecting and analyzing the CL signal in an electron microscope, this powerful technique combines the functional optical information of luminescence spectroscopies with the high spatial resolution of electron microscopy. This makes CL an attractive technique for a wide variety of applications and research, especially in optics research, materials science, and geology.
In an electron microscope, you can attain spatially resolved information (images or maps) by scanning a sub-nm diameter electron beam across the surface of a sample. By collecting and analyzing the CL signal in an electron microscope, this powerful technique combines the functional optical information of luminescence spectroscopies with the high spatial resolution of electron microscopy. This makes CL an attractive technique for a wide variety of applications and research, especially in optics research, materials science, and geology.
Webinar
Cathodoluminescence Explained. Episode 1: An Introduction